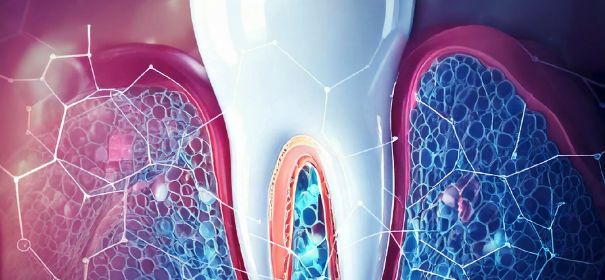
SyrHA offers the manufacturing of one batch in its facility to let you carrying out your first physico-chemical and bio compatibility tests.
Terms
- You present an abstract of your medical device or pharmaceutical product (composition, use…)
- Your product is composed of at least one water-soluble biopolymer (e.g. HA, chitosan, agarose, etc.) you send your application with our contact form before 31/12/2023.
SyrHA and ISHAS’s teams will meet to choose the best project. The winner will be revealed during the ISHAS 2024 event and the production of the first batch will be launched in the 2nd half of 2024.
"*" indicates required fields
Turning your idea into reality
We support you from the initial idea to the manufacturing of the product, whether it is :
- an injectable dermal filler
- a viscosupplementation device
- an ophtalmic device
- or an innovative hydrogel
-
Our ready-to-use formulations
Do you need to save time ?
SyrHA proposes several ready-to-use formulations that meet your needs.
Consult our portfolio of formulations and check our product specifications and features for dermal fillers, OVDs and viscosupplements. -
Manufacturing Activity
Do you have a developed formulation and now you are looking for a qualified manufacturer?
SyrHA can manufacture in its facility your future product according to your formulation, your requirements and the capacity you need. As part of your project development, we can manufacture small batches for preclinical and clinical evaluations. -
Technological Transfer
Do you need to manufacture your product into your facility?
SyrHA can support with the implementation of a qualified and validated manufacturing factory :
– plant gap analysis
– technical development
– formulation manufacturing
– validation activies